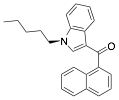
Answers:
1. What
is the molecular formula for JWH-018?
C24H23NO
2. How
many of the atoms in JWH-018 are sp-hybridized? sp2? sp3?
sp: 0
sp2: 19 carbons; the oxygen is also sp2
sp3: 5 carbons; the nitrogen is also sp3
3. What
is the formal charge of the nitrogen atom?
Nitrogen is in column 5.
It has 3 bonds (6 electrons) and 1 one non-bonded pair of
electrons to complete the octet.
Calculating: column – bonds – non-bonded electrons:
5 – 3 – 2 = 0
The nitrogen atom has a formal charge of 0.
4. What
is the correct geometry for the nitrogen atom? Is it drawn accurately?
3 atoms directly attached to the N
plus 1 non-bonded pair (omitted from the drawing)
3+1 = 4; sp3 which means tetrahedral geometry
No, the drawing is not three
dimensionally accurate. The bond of the chain on the left would be drawn either
into the paper away from us or out of the paper toward us. For example: