

The light is needed in this reaction to break the π bond between the carbon and oxygen in the carbonyl group of benzophenone forming a diradical. Of the bonds in the two reactants, this bond is weakest making it most susceptible to the energy provided by the ultraviolet light.

After the diradical forms it immediately becomes a scavenger, its oxygen atom quickly finding a relatively easily removed hydrogen in the 2° position in the plentiful solvent molecules. In this step the diradical becomes a benzhydrol radical, while the solvent molecule becomes a new radical.
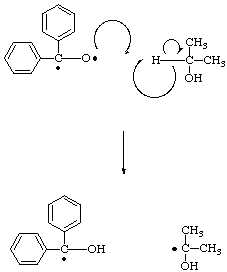
This newly formed radical is now highly reactive, enabling it to offer a hydrogen atom to the oxygen of another benzophenone diradical, thus resulting in the formation of a stable molecule, acetone, as well as another benzhydrol radical.

Eventually one benzhydrol radical will collide with another leading to the formation of the final stable product.

Procedure
Place 2 g benzophenone into a 20 mL vial and add about 10 mL 2-propanol.
Dissolve the solid with gentle warming in a warm water bath. After the solid
is dissolved, add 1 drop glacial (100%) acetic acid, then fill the vial with
2-propanol
until it is a little more than half way into the screw neck of the vial. Tightly
fasten the cap and invert the vial several times to mix the liquid. When the
vial is inverted, if more than a small bubble of air is present, reopen the
vial and add a little more 2-propanol.
Wrap the cap with a small strip of Parafilm to avoid any leakage, taking care not to wrap Parafilm around the main body of the vial. Put a strip of lab tape on the top of the vial with your name, the date, and the name of this laboratory. Place the vial in the windowsill in such as way that it will be exposed to as much light as possible.
Next week, harvest the product crystals which form by pouring off the liquid (organic waste container) and scraping the crystals from the vial with a spatula. After the crystals dry on an open watch glass, determine their mass and melting point.
Prelab Questions
Use online (or other) sources to answer the following questions prior to laboratory.
| 1. Using molecular weight, determine the number of millimoles of benzophenone used. |
| |
| 2. Using density and molecular weight, determine the number of millimoles of 2-propanol used. |
| |
| 3. Determine the mass (in milligrams) of product expected. |
| |
|
4. Find a literature melting point for the product, benzopinacol Include reference to source. |
| |
|
5. Why are you asked to "take care not to wrap
Parafilm around the main body of the vial? |
|
Literature Sources
Organic Syntheses (online), Photochemical reactions
Norrish type cleavage reactions
Aldrich Chemical Company Search Page
ChemExper Chemical Directory
Acros Chemicals
NIST Chemistry Webbook
References
University of Manitoba Department of Chemistry: Chemistry 2.339
Organic Syntheses (online), Collective Volume 2
Experimental Methods in Organic Chemistry, (3/e), Moore/Dalrymple/Rodig, Holt, Rinehart & Winston, Inc., 1982.