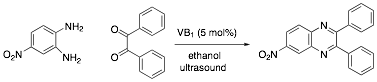
Green synthesis of
6-nitro-2,3-diphenylquinoxaline [CAS 7466-45-7]
In this
laboratory, we will prepare an aromatic heterocyclic molecule from the class of
molecules called quinoxalines. These rings are rare
in nature; however, they have been found to be useful products for medicinal
purposes.
In this
laboratory experience you will examine the synthesis of heterocyclic molecules.
In addition you will see some ways that green chemistry principles can be used
to prepare the products more safely than has been done previously. In specific
you are using a safe substance (thiamine, Vitamin B1) as a catalyst. We are
also speeding the reaction by the use of ultrasound. Lastly, the published
procedure from which this method was derived uses more hazardous solvents and
produces a larger amount of waste.
QUESTIONS
1. Convert the
amount of diamine and diketone
from moles to mass.
2. Calculate the
amount of thiamine needed; multiply the number of moles of the limiting reagent
by 5% and convert from moles of thiamine to mass of thiamine.
3. Go online to
find the Twelve Principles of Green Chemistry. List the principles that this
laboratory seems to incorporate.
PROCEDURE
In a standard
sized test tube, dissolve 4-nitro-o-phenylenediamine
(1.1 mmol), benzil (1 mmol), and thiamine catalyst (see question above) in 5 mL
of ethanol. Clamp the test tube into an ultrasonic bath at room temperature and
sonicate for 1 hour. Add 10 mL water to the resulting
mixture. Chill, stir well and collect the product by vacuum filtration. If
the product is orange, it can be purified by recrystallization from 70% ethanol.
After the product has dried obtain IR spectrum and melting point (literature value =
193-195 °C).
REFERENCES
Aghapoor, K.; Mohsenzadeh,
F; Talebian, S.; Tehrani,
M. J.; Balavar, Y; Khanalizadeh,
G.; Darabi, H. R. Vitamin B1 as a
metal-ion-free natural catalyst for sustainable quinoxaline
ring condensation under sonochemical conditions. Montash. Chem. 2011, 142,
619-624. link
Mohsenzadeh, F; Aghapoor,
K.; Darabi, H. R. Benign Approaches for the
Microwave-assisted Synthesis of Quinoxalines. J.
Braz. Chem. Soc. 2007, 18,
297-303.