
Aldersey-Williams, Hugh. The Most Beautiful Molecule: the discovery of the buckyball. New York: John Wiley & Sons, 1995. p. 58
Abstract:
Since its Nobel-Prize-winning discovery in 1985, many new findings have been made concerning Buckminsterfullerene, which is also known as the C60 fullerene or the buckyball. It is a hollow sphere of carbon atoms, with pentagon faces that are completely surrounded by hexagons, and resembles a soccer ball.
Buckminsterfullerene is fully conjugated and was once thought to be aromatic, but its bonds are actually partially localized. This lack of aromaticity is manifested in its ability to undergo addition reactions. It is highly stable because it fills the valences of all 60 carbon atoms, has partially delocalized pi bonds, and meets the isolated pentagon rule.
Many applications are currently under investigation. Its metal-containing derivatives are of special interest in the area of superconductivity. The hollow space inside C60 is big enough for an atom or very small molecule, so buckyballs possibly could be used to transport medicines in the body.
Outline
Buckminsterfullerene: An Overview
"THEY CAME FROM OUTER SPACE!" papers say (1). But the papers are not just tabloids in a supermarket aisle, and reporters are not talking about flying saucers. Instead, the commotion is about something too small for even an electron microscope to detect: molecules trapped in old meteorites. Yet these molecules’ probable extraterrestrial formation is not what has made them famous. (1,2)
Rather, what makes this molecule so unique is that it is a hollow sphere of sixty carbon atoms, a frame of hexagons surrounding pentagons, making it look like a soccer ball. Its class of molecules is the only known form of pure carbon besides diamonds and graphite. This molecule is often referred to as a "buckyball" in lieu of their twenty-letter, six-syllable title–"Buckminsterfullerene", which was bestowed upon it in honor of Buckminster Fuller, the architect who invented geodesic domes. After the discovery of buckminsterfullerene, scientists began discovering other similar molecules. They called this class of molecules fullerenes. (3)
The possible applications for buckminsterfullerene are endless. Compressing them results in a material even harder than diamonds (4). Fullerene-like tubules may be 100 times stronger than steel, making the strongest known substance (5). There are even reports buckyballs can be used to deactivate viruses (6), and there is significant data suggesting that they could someday be used to safely transport chemotherapy to targets within the body (7,8).
DISCOVERY
 |
|
Figure 1. The laser vaporization experiment performed by Kroto, Smalley, Curl, Heath, and O’Brien. Aldersey-Williams, Hugh. The Most Beautiful Molecule: the discovery of the buckyball. New York: John Wiley & Sons, 1995. p. 58 |
The experiment transformed graphite into carbon soot. A laser beam vaporized the graphite by heating it to over ten thousand degrees Celsius–above the surface temperature of most stars. Next, a burst of gas ushered the vapor into a vacuum chamber, where it cooled to only a fraction of a degree above absolute zero. The vapor condensed and formed clusters of carbon atoms. In order to measure the size of these clusters, another laser pulse hit the clusters and ripped electrons from them, making them partially positive. An electric field drew the positively charged clusters toward a detector. Lighter molecules traveled faster than heavy ones; therefore, it was possible to determine the cluster’s atomic weight by the amount of time it took them to get to the detector. [See Figure 1] (10)
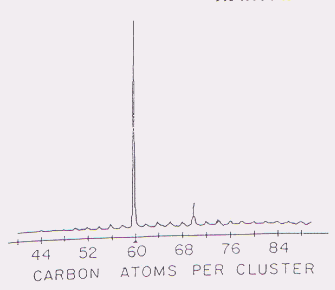
The molecule that contained sixty carbon atoms was conspicuously plentiful in this laser-created soot. [See Figure 2]
 |
|
Figure 2. The peak in C60 yield at optimal conditions. Curl, R.F. "Pre-1990 evidence for the fullerene proposal"The Fullerenes. New York: Pergamon Press, 1993. p. 13 |
In the November 1985 issue of Nature, these scientists announced their discovery of Buckmisterfullerene–a highly stable sphere of 60 carbon atoms, arranged in hexagons and pentagons, with alternating double and single bonds. [See Figures 3 and 4] While the article was only two pages and contained some errors in regards to the molecule’s physical properties, its scientific importance cannot be underestimated; it has since been cited in almost every book or article written about this class of molecules. (11,12)
After the announcement of the C60 fullerene’s discovery, someone unearthed a 1970 theoretical chemistry paper written in Japanese. In it, Eiji Osawa predicted the existence of C60 as a superaromatic molecule. The article had not originally attracted much attention because of the language barrier. However, now scientists were excited about what they could do with a superaromatic sphere of carbon atoms. (12)
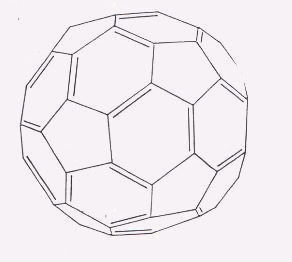 |
| Figure 3. A buckminsterfullerene bond-line drawing. Curl, R.F. "Pre-1990 evidence for the fullerene proposal" The Fullerenes. New York: Pergamon Press, 1993. |
C60: AN AROMATIC DISAPPOINTMENT
Buckminsterfullerene is completely conjugated. Therefore, scientists originally speculated that its pi bonds would be fully delocalized, making it a "superarene." That assumption seemed plausible since C60’s delocalization energy per carbon exceeded that of benzene, and it had as many as 12,500 Kekulé structures. Furthermore, it’s 13C NMR shift was between 142.5 and 143.2, which is similar to that of aromatic hydrocarbons that suffer significant ring strain. (3,13)
However, one of Hückel’s requirements states that a molecule must be flat in order to be aromatic. Buckminsterfullerene is not. The angle between pentagons and hexagons causes the pi bonds to be partially localized. Consequently, there are two classes of bonds: "single" bonds where hexagons and pentagons meet, and "double" bonds between two hexagons. Due to partial delocalization, the actual bond orders are 1.476 and 1.601, and the bond lengths are 1.458 A and 1.401A respectively. Buckminsterfullerene is not aromatic; in fact, it is no more aromatic than [8]-annulene. (3,13,14)
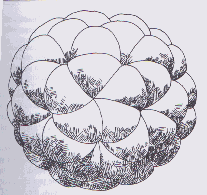 |
| Figure 4. A more realistic representation of buckminsterfullerene Aldersey-Williams, Hugh. The Most Beautiful Molecule: the discovery of the buckyball. New York: John Wiley & Sons, 1995. p. 79 |
STABILITY
Although buckyballs are not aromatic, there are several factors that contribute to their unusual stability. Here are the most important of these:
A. Pentagons
All fullerenes are made of only five- and six-membered carbon rings. In order to close into a sphere, every fullerene must have exactly 12 pentagons, but the number of hexagons can vary. Therefore, fullerenes are possible for any even number of carbon atoms greater than or equal to 20 except 22. This is why in their original experiment, Smalley, Kroto, Curl, Heath, and O’Brien noticed that large carbon clusters only formed for even numbers of carbons. (10, 15, 16)
The isolated pentagon rule states that fullerenes are most stable when all of the pentagons are completely surrounded by hexagons. There are two main reasons for this: first, there is increased angle strain on carbon atoms that are forced to be part of two or more pentagons; this strain destabilizes the molecule. (15)
The second reason that fullerenes with isolated pentagons are more stable has to do with conjugated circuits in resonance structures. A conjugated circuit is a sequence of alternating double and single bonds around the edge of a ring of similarly sized atoms. A ring that contains six pi electrons is referred to as a 6-circuit, and it is the most stable. 10-circuits are also unusually stable, but not as stable as 6-circuits. A circuit can also go around the perimeter of two rings that share a side. When pentagons are isolated, only conjugated 6- and 10- circuits occur. However, abutting pentagons result in 8-circuits that destabilize the resonance structure. (15)
Buckyballs consist of 12 pentagons and 20 hexagons. They are the smallest fullerene that fulfills the isolated pentagon rule, making them especially stable (15).
B. Sphericity and Symmetry
Fullerenes that are spherical, like C60, rather than oblate are more stable because the angle strain is distributed more evenly (15). X-ray diffraction from solid C60 has confirmed its high symmetry, and C60’s single sharp resonance in 13C NMR indicates equivalence of all its carbons (18). Also, all carbons have the same kind of bonds–two "single" bonds and one "double". Since all the carbon atoms are equivalent, each one bears an equal amount of strain. There are no self-evident reactive sites; hence, the molecule is more stable. (15)
C. Charge
Buckminsterfullerene is very polarizable because it contains numerous pi electrons, which are delocalized about the molecule. It can also can gain or lose electrons, becoming a negative or positive ion. C60 ions are unusually stable because of their high degree of delocalization (19). However, they get distorted as they lose electrons, and losing too many electrons will cause them to break apart (3).
ENDOHEDRAL METAL COMPLEXES
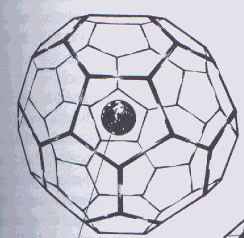 |
| Figure 5. A lanthanum atom inside a carbon cage Aldersey-Williams, Hugh. The Most Beautiful Molecule: the discovery of the buckyball. New York: John Wiley & Sons, 1995. p. 97 |
Despite the limited space inside buckyballs, medical researchers are hoping to use them to encapsulate and deliver powerful drugs–such as radioactive metal ions in cancer treatment–to specific locations in the body. In order to make the buckyballs target a certain tissue, a functional group that is attracted to that tissue can be attached to the outside of C60’s cage; for example, researchers have shown that biphosphonated buckminsterfullerene is attracted to bone. (8)
One advantage of using buckyballs as transporters is that they resist breakdown in the body, making them good for carrying dangerous compounds that would wreak havoc if the cage were to split open prematurely. However, this also raises questions about how easily buckyballs could be flushed out of the body. (8)
REACTIONS
Buckminsterfullerene is not aromatic. Buckyballs behave much more like an alkene and readily undergo additions. However, multiple additions usually occur, and the reactions are not regioselective (21). This creates a complex mixture of products and isomers that is difficult to analyze (23).
A variety of nucleophiles, including both dipoles and anions, can add to buckyballs. This reaction begins by electron transfer to a carbon on the C60 molecule. A covalent bond forms between the nucleophile and the carbon. This overfills the carbon’s valence and causes its double bond to break. (22) Here are a few examples of nucleophilic substitution reactions:
room temp
C60 + RNH2 ---------------> C60 (NH2R)x "x" varies depending on R:
THF MeI
C60 + Ph:MgBr -----------> ---------->
C60Ph10Me10
THF MeI
C60 + Ph:Li -------------> -----------> C60PhxMey x = 2,1,0; y = 3,2,1 (22)
The C60 fullerene is electronegative and a mild oxidizing agent. It is an excellent electrophile, so it can be added to aromatic molecules in Friedel Crafts Fulleration. It is also a good dienophile and can undergo Diels-Alder cycloaddition. (21,22)
Buckminsterfullerene acts like a sponge to free radicals. These radicals can be generated in a number of ways, including light- and heat-initiated free radical reactions. (23)
A. Metalation
Functional groups containing metal atoms can be attached to buckyballs. These groups lower the electron affinity of the fullerene and make the addition of each successive metal atom more difficult. This means that the reactivity of fullerenes can probably be controlled by the number of metal-containing functional groups that are added. Researcher are trying to verify this. (23)
Some metal-doped fullerenes are superconductors, meaning that they conduct electricity with no energy loss caused by resistance. However, in order to lower a material’s resistivity and make it a superconductor, it must be cooled to near absolute zero. Much research has been done to find compounds that are superconductors at higher temperatures. Fullerenes with metal atoms attached to them become superconductors at greater temperatures than most other superconducting materials, and they are also easier to make than most. The metal atom does not have to be directly attached to the fullerene cage. For example, in the case of the osmium tetraoxide adduct (t-BuC5H4N)2OsO4C60, the metal ion is indirectly attached to the buckyball via two oxygens. (4, 23)
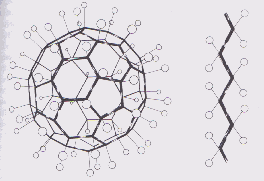 |
| Figure 6. Right: a flourinated buckyball; Left: a Teflon molecule Aldersey-Williams, Hugh. The Most Beautiful Molecule: the discovery of the buckyball. New York: John Wiley & Sons, 1995. p. 247 |
Buckminsterfullerene readily undergoes oxidative halogenation with Fl, Cl, and Br, so the majority of the product is polyhalogenated (21,23). Florinated C60 resembles Teflon. [See Figure 6] Some scientists therefore had hoped that it could be used as a lubricant, but tests have proved unsuccessful, and most scientists now feel that it is a dead end (4).
LOOKING TOWARD THE FUTURE
Years have passed since the discovery of Buckminsterfullerene. Buckyballs are some of the most researched organic molecules in history. Now people, especially those footing the bill, want to see results. Even Richard Smalley, one of Buckminsterene’s co-discoverers, is growing impatient, and he says:
This kid shouldn’t be living with his parents anymore; he should be out there with his own job. But some degree of caution is called for in making big-time applications for Bucky. On the other hand, this isn’t any old molecule. This is a molecule built out of carbon. Carbon is by far–big time far–more versatile in its chemical bonding than any other element. (5)
Although the ideas for Buckminsterfullerene’s practical applications are endless, these applications have yet to journey from the imagination to reality. That will take more time. However, the science world agrees almost unanimously that such an unusual class of carbon molecules will eventually be used for something revolutionary. (4)
Aldersey-Williams, Hugh. The Most Beautiful Molecule: the discovery of the buckyball. New York: John Wiley & Sons, 1995.
This book has a conversational tone that is very easy to understand. A large portion of it is history that would fit better into the introduction than the main discussion. It also describes a variety of applications for C60. There is a chapter entitled "The Chemical Senses" that discusses spectrometry, and there are random bits of scientific detail and description scattered throughout the text.
Baggot, Jim. Perfect Symmetry: The Accidental Discovery of Buckminsterfullerene. New York: Oxford University Press, 1994.
This will be good for the main body of my paper. It contains information on C60’s electron arrangement, properties as a mild oxidizing agent, alkene-like reaction with hydrogen, altered stability when contains a metal atom inside its cage, and possible mechanism of formation.
Bortz, Fred. "Finding a job for bucky: and interview with Richard E. Smalley," Odyssey v6 (iss1) p 22-3.
This is cute and has a lot of great quotes. I used one of the quotes in my conclusion.
Cioslowski, Jerzy. Electronic Structure Calculations on Fullerenes and Their Derivatives. New York: Oxford University Press, 1995.
Chapter 3 of this book is entirely devoted to Buckminsterfullerene, C60. It contains sections on geometry, bond length, vibrational frequencies, heat of formation, orbital energy levels, ionization potentials, electron affinities, positive and negative ions, excited states, electric polarizabilities, the 13C NMR spectrum, and a possible mechanism of formation. There is also a sixteen-page bibliography (just on chapter 3!!!).
Curl, R.F. "Pre-1990 evidence for the fullerene proposal" The Fullerenes. New York: Pergamon Press, 1993
The author is one of the original discoverers. This gives the scope of fullerene research prior to 1990.
Fagan, Paul J., Joseph C. Calabrese, Brian Malone. "The Chemical Nature of C60 as Revealed by the Synthesis of Metal Complexes," Fullerenes: Synthesis, Properties, and Chemistry of Large Carbon Clusters. Washington, D.C.: American Chemical Society, 1992: 178-86.
I can use this article as evidence when talking about how C60 fullerene is not aromatic since its pi bonds "behave like those in electron-poor arenes and alkenes" (184).
Fagan, Paul J., Bruce Chase, Joseph C. Calabrese, David A. Dixon, Richard Harlow, Paul J. Krusic, N. Matasuzawa, Frederick N. Tebbe, David L. Thorn, E. Wasserman. "Some well-characterized chemical reactivities of buckminsterfullerene," The Fullerenes. New York: Pergamon Press, 1993: 75-99.
Fowler, P.W., D.E. Manoupoulos. An Atlas of Fullerenes. New York: Oxford University Press, 1995.
This mostly talks about fullerenes other than buckminsterfullerene. However, there are four pages (p 44-7) that tell interesting things about C60. It tells about bond order, partial localization, and how it is more like an alkene than an arene.
Graham, David. "They came from outer space," Earth v5 (iss5) p 13.
Haddon, R.C., K. Raghavochari. "Electronic Structure of the Fullerenes: Carbon Allotropes of Intermediate Hybirdization," Buckminsterfullerenes. New York: VCH Publishers, 1993: 185-199.
Hammond, George S. "Fullerenes: Overview 1991," The Fullerenes. New York: Pergamon Press, 1993: ix-xiii.
Heath, James R. "Synthesis of C60 from Small Carbon Clusters: A Model Based on Experimental Theory," Fullerenes: Synthesis, Properties, and Chemistry of Large Carbon Clusters. Washington, D.C.: American Chemical Society, 1992: 1-23.
The author is one of the original discoverers and is therefore noteworthy. This article presents one possible mechanism of formation for fullerene from graphite. This might be useful in a "possible mechanisms of formation" section in my paper, but it also might be too in depth.
Henderson, Alan D. "Viricidal," Blood Weekly 5/26/97 p26
This is another interesting application for buckyballs, and I’ll use it in my introduction.
Koruga, Djurko, Stuart Hameroff, James Withers, Raoulf Loutf y, Malur Sandershan. Fullerene C60: history, physics, nanobiology, nanotechnolog. Netherlands: Elsevier Science Publishers, 1993.
This was written by people who have made relatively significant discoveries about fullerenes, and so it contains a lot that would be relevant to the main body of my paper. However, its tone seems cheeky, arrogant, and intentionally irreverent to God. Therefore, I’ll probably avoid using this source.
Kroto, H.W., H.R. Heath, S.C. O’Brien, R.F. Curl, R.E. Smalley. "C60: Buckminsterfullerene," Nature v318 p162-3.
This article is very famous. It’s the original paper that the discoverers published to announce their findings. Apparently, it contains some minute errors. However, it is included in the bibliographies of any major papers written about fullerenes.
"Laser-created buckyballs earn discoverers Nobel Prize in Chemistry," Laser Focus World. V 32 (iss 11) p 9
Again this would be good in the introduction because it tells that Harold Kroto, Robert Curl, and Richard Smalley won the ’96 Nobel Prize for their discovery of buckyballs.Nemecek, Sasha. "A tight fit," Scientific American. V 273 (iss 4) p 34.
Probably only good for my introduction. This is a short article telling how buckyballs can be pried open and reclosed, and how researchers hope that they will be able to stuff something inside. However, the space inside buckyballs only big enough for a single atom or very small molecule.
Olah, George A. "Chemical reactivity and functionalization of C60 and C70 fullerenes," The Fullerenes. New York: Pergamon Press, 1993:
This article was useful in the discussion of C60’s reactions. I used it for the main body of my paper.
Schmalz, T.J., D.J. Klein. "Fullerene Structures," Buckminsterfullerenes. New York: VCH Publishers, 1993: 83-101.
Shlüter, M. "Superconductivity in alkali intercalated C60," The Fullerenes. New York: Pergamon Press, 1993.
Solomons, T.W. Graham. Fundamentals of Organic Chemistry. New York: John Wiley & Sons, 1997: p 592
This is less than a page, and some of it is outdated. However, it’s a good starting point for understanding the basics of what fullerenes are.
Wang, C.Z., B.I. Zhang, C.H. Xu, C.T. Chan, K.M. Ho. "Structure and Stabilities of Carbon Fullerenes," Clusters and Fullerenes: Proceedings of the Adriatico Research Conference, June 1992. Singapore, World Scientific Publishing Co., 1993: 249-253.
This discusses the factors that contribute to fullerene stability. It states that 60, 70, and 84 are the "magic numbers" of carbons that give stability in a fullerene. However, it does not say much more than this, and therefore the usefulness of this article might be limited.
White, C.T., J.W. Mintmire, R.C. Morwrey, D.W. Brenner, D, H, Roberson, J.A. Harrison, B.I. Dunlap. "Predicting Properties of Fullerenes and their Derivatives," Buckminsterfullerenes. New York: VCH Publishers, 1993: 125-181 .
Wu, C. "Altered Buckyballs go straight to bone," Science News. V 155 (iss 19) p 292.
This would be interesting in my introduction. It tells how scientists anticipate using fullerenes to deliver drugs to diseased tissue.
Wudl, Fred. "Chemistry of Fullerenes," Buckminsterfullerenes. New York: VCH Publishers, 1993: 317-333.
This guy appears to be the expert on fullerene reactions. He has written the reaction chapter in two of the books I looked at. I used this in the main body of my paper.
Wudl, F., A. Hirsch, K.C. Khemani, T. Suzuki, P.-M, Allemand, A. Koch, H. Eckert, G. Srdanov, H.M. Webb. "Survey of Chemical Reactivity of C60, Electophile and Dieno-polarophile Par Excellence," Fullerenes: Synthesis, Properties, and Chemistry of Large Carbon Clusters. Washington, D.C.: American Chemical Society, 1992: 161-75.
Obviously, I would use this in the main body of my paper, as a major source in a discussion of fullerene’s chemical reactivity.
Zimmer, Carl. "Buckyballs from space," Discover. V17 (iss 8) p 30.
This article would probably work delightfully in the introduction. It tells how large quantities of fullerene was found in a meteorite.