Abstract
Microencapsulation is a technique borne out of colloid chemistry — the chemistry of substances that are suspended, but not dissolved, in a given solution. First made useful in the early 1940’s, the past nearly sixty years have led to innumerable applications for the relatively simple concept.
At its most fundamental level, microencapsulation (also known as coacervation) is nothing more than forming a membrane of any kind around any very tiny particle, liquid or solid. But the simple idea of microencapsulation has led to a complex, extensive field of study and applications in nearly every area of life, from scratch ’n’ sniff stickers to cancer treatments and from pesticides to paints.
Outline
Microencapsulation — How it All Began
Do you remember bringing home that paper with a sticker that smelled like ice-cream every time you scratched it, even months later? While the fad of scratch ‘n’ sniff stickers was sweeping through America’s eighties generation, the same technology that made scratch ‘n’ sniff stickers possible was having a much more significant effect among the "grown-up" world.The story began almost half a century earlier, in the late 1930s, when young chemist named Barret K. Green went to work for the National Cash Register (NCR) Corporation. Green was especially interested in the chemistry of colloids - particles that don’t dissolve in solution, but can be dispersed into droplets tiny enough to be almost considered "in solution." It was a field that up to that time had seen little useful application. But when Green’s company needed a way to produce multiple copies of printed pages that didn’t require the use of messy carbon paper, Green remembered the work of a chemist named Bungenburg de Jong. De Jong had written papers describing methods for using colloid solutions to form a solid coating of gelatin around droplets of an oily liquid. By 1942, Green had taken this method and invented a way to apply de Jong’s technique to the formation of solid, dye-containing capsules that could be mixed with an adhesive and painted onto a piece of paper. These tiny capsules were designed in such a way that they would break under pressure, releasing the dye to react with a coating on the paper below and creating a dark mark in exact copy of the marks on the paper above.1
Green’s tiny capsules became known as microcapsules or microspheres and the process of their formation as microencapsulation. Green’s particular process of creating a colloidal solution of the core material (in his case, the dye) in order to form a coating around it was referred to as coacervation. While capsule formation had been an area of scientific interest for decades, coacervation produced capsules that were smaller and more evenly coated than any spraying technology at that time could produce. These new microcapsules sparked the interest of scientists and industries across the nation. As the ‘40s rolled into the ‘50s, and then the ‘60s, Green’s coacervation microencapsulation grew from a simple method of producing carbon copy paper to a worldwide industry. Soon, solids and gases, as well as liquids, were being enclosed in tiny microspheres. Companies like 3M (the producer of Scotch tape and other products) 2 and the Southwest Research Institute (SwRI)3 began adding their patents to the growing list of microencapsulation techniques. New companies grew up out of the technology itself, specializing in providing microencapsulation services for their customers.4
In the 1960’s, the SwRI added a new technique to the encapsulation market. Known as co-extrusion, this technique used an apparatus called a drip jet which contained a core nozzle surrounded by a circular arrangement of coating nozzles. The apparatus was designed to release core and wall materials at the same time, resulting in drops of core material being coated as they dripped from the nozzle (Figure 1).
 |
Meanwhile, other researchers were developing ways to increase the strength of the capsule walls once they were formed. Walls made of waxes and fats were easily hardened by cooling, but re-heating such capsules resulted in instant melting of the wall. Scientists soon found that various polymer cross-linking techniques provided an easy, adjustable way to harden a capsule wall. Cross-linking, a usually irreversible reaction, involved linking individual polymers by a network of new covalent bonds, hydrogen bonds and ionic attractions.
As the research picked up pace, chemists discovered that certain microcapsule wall materials could be induced to "leak" their contents slowly, over a period of time, rather than simply when the capsule wall was broken under pressure, melted by heat or dissolved in water. Soon, the applications ranged from scent and flavor encapsulation - which led to applications in food products, magazine perfume inserts and eventually, by the late ‘70s, an exploding scratch ’n’ sniff sticker industry - to such diverse products as pesticides, detergents, fertilizers, food preservatives, kitty litter, razor blades, facial tissue and medicines.
Why Microcapsules?
Microcapsules proved useful for a number of reasons. First of all, they were relatively small. Ranging (by definition) from approximately 1m m to 1000 m m in diameter, they could enclose tiny amounts of core material within a large surface area, increasing surface area to volume ratios dramatically. When microcapsules were used to enclose medicines, pesticides or other materials that required a slow, sustained, delivery, this high surface to volume ratio allowed for a quicker diffusion of the contents as well as a a higher percentage of core material release compared to large capsules. Secondly, microcapsules could change the apparent properties of the particular core material. Encapsulation turned liquids into fine-grained powders. Hydrophilic substances could be made to be hydrophobic, and vice versa. Fragile core materials could be protected from their environment, and toxic substances made safe to handle and store. Finally, the capsules could be designed to release their contents in a variety of ways - total rupture (chemical, temperature or pressure induced), quick diffusion or prolonged release, lasting anywhere from days to months — simply by the design of the microcapsule wall.
It does not take much creativity to imagine the vast range of applications for such a technology. For the past fifty years, some of the top minds in applied chemistry have been paid both to discover new ways to make capsules as well as new things to enclose in them. Today, there are nearly as many ways to encapsulate a substance as there are applications for that encapsulation. But how does this process work?
The Process of Microencapsulation
There are two important questions scientists must ask when considering microencapsulation. First, what type of material do they want to enclose? And second, what properties does the wall need to have? Which method may be the most appropriate to use depends on a number of factors, but if the coacervation method is to be used, the most important factor is that of solubility. To achieve an effective coacervation reaction, the core material must be able to be suspended — but not dissolved — in the chosen solvent, while the wall material(s) must at first be able to be dissolved in the solvent and then, later, induced to come out of solution in such a way as to adhere to the suspended particles of core material in the process.
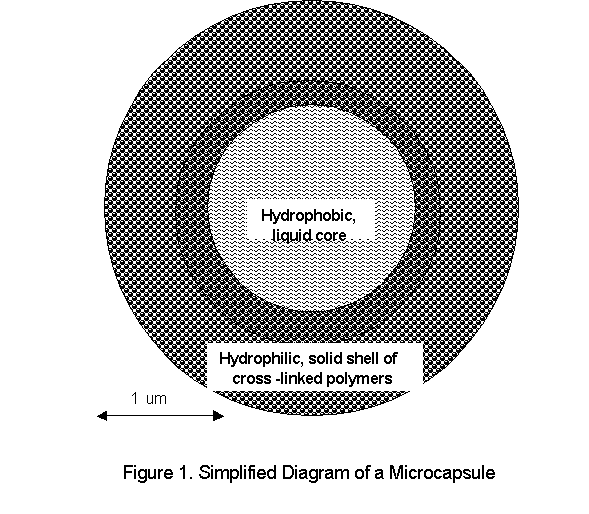
Since microencapsulation often is performed to encase hydrophobic particle in a hydrophilic sheath (Figure 2),
 |
| Figure 2: A drip jet co-extrusion apparatus from Southwest Research Institute brochure, October 1990, Southwest Research Institute, San Antonio, Texas. |
The oil in water method came first, and is the method used most often in food and fragrance applications. Often, the flavor and fragrance molecules are hydrophobic organic molecules that need to be able to dissolve effectively in water, and microencapsulation makes this possible. The simplest method begins by dissolving the gelatin (or another hydrophilic polymer that is to become the wall material) in water and adding the core material, while maintaining the appropriate temperature and stirring speed. The hydrophobic core material will not dissolve in the water. However, the agitation created by the stirring will disperse the core material into tiny particles that will be suspended throughout the solvent. Once this state is achieved, the wall material must be brought out of solution in such a way that it will coat the dispersed particles of core material. This can be accomplished by adding a strongly hydrophilic material (more hydrophilic than the gelatin), such as sodium sulfate, by changing the pH of the solution or by adding a hydrophobic, miscible material like ethanol. Each of these options will cause the solution to separate into two different phases that, while both aqueous, cannot mix. With proper manipulation of the phase separation, the wall polymer becomes concentrated in only one of the two phases and is virtually absent, or at least in very low concentration, in the other. This sudden concentration of the wall material causes the polymer to coagulate (clot up), adhering to and coating the particles of core material in the process. Occasionally, a particular wall material can be effectively brought out of solution and made to coagulate simply by a change in temperature. The entire system is then cooled, and the microcapsules are collected by filtration, washed with water, and dried. The result is a dry, hydrophilic, gelatinous capsule containing hydrophobic core material. The applications of this method can be especially important to medicine because the resulting powder can be dissolved in an appropriate aqueous solution and injected into the bloodstream, which, being hydrophilic itself, will readily carry hydrophilic microcapsules but would have difficulty transporting oily, hydrophobic capsules.
One of the simplest examples of this method is the formation of drug-containing albumin microspheres. In this particular procedure, albumin, a protein common in the watery fluid of the blood, forms a hydrophilic coating around the hydrophobic drug. Because of its compatibility with the human immune system human serum albumin (the human version of albumin) is a favorite for immunological applications of microsphere formation. While albumin is such an important drug carrier that numerous techniques have been developed, most useful techniques are variations of the procedure shown here for creating human serum albumin microcapsules around a core of 5-fluorouracil (a drug).
The procedure begins by dissolving one gram of human serum albumin (albumin from human blood fluid) in 2.0 mL of deionized water by agitation with a magnetic stirrer. Next, 0.1 g of 5-fluorouracil is added to the solution, and the solution is stirred for an additional 15 minutes. While this is being done, 500 mL of cottonseed oil is placed in a 600 mL stainless steel beaker at room temperature and stirred continuously at 2300 rpm by a 1.5 inch propeller style stirrer. A special needle is then used to inject the albumin solution into this prepared cottonseed oil, which will act as a heating bath for the aqueous albumin solution. Stirring is continued for the next 15 minutes while the temperature of the oil bath is raised with a 500-W immersion heater to a temperature of 140 degrees. The bath is stirred at this temperature for one hour. In this procedure, the heat, rather than a chemical reaction, induces coagulation of the albumin protein, which forms cross-linking bonds (hydrogen, disulfide and/or ionic bonds between proteins), resulting in a matrix-like coating of albumin over the 5-fluorouracil core material. Once the hour has passed, the oil bath and its contents are cooled to room temperature, and the microcapsules are filtered out of the solution by vacuum filtration with Whitman No. 5 filter paper. Lingering traces of oil are removed by washing the microspheres several times with 30 mL rinses of heptane. The result of this fairly simple procedure is a free-flowing, light tan powder, consisting of 10-60 m m microcapsules containing 9.6% weight/weight of the encapsulated drug.6
While this procedure itself is quite simple, it is also limited in scope, and has several problematic results. Numerous variations of this method have been developed in response to these results, as well as to meet other very specific needs. Several of these issues, and their respective variations, will be discussed later.
The water in oil method was developed after the oil in water technique, but it too has become important in many applications. In this method, the core material is dispersed in an organic, non-polar solution (cyclohexane, etc.) containing the hydrophobic wall material (a hydrophobic polymer like ethyl cellulose). As before, once this suspension is obtained, the wall material must be somehow brought out of solution. This can be accomplished in several ways. One method is to heat the resulting solution and then quickly cool it again, bringing the wall material out of solution and forcing it to coagulate. Alternatively, the wall polymer can be chemically induced to coagulate by the addition of an organic (such as polyisobutylene or polyethylene) that is even more soluble in the medium than is the cell wall material. As the added organic dissolves, the wall material becomes increasingly less soluble, eventually coming out of solution and adhering to the suspending particles of core material. The solution is then cooled to allow the microcapsules to harden, rinsed with an appropriate organic solvent, and dried. The result is usually a dry, powdery product of hydrophobic microcapsules containing either hydrophobic or hydrophilic core material(s).7
Variations in Microencapsulation Coacervation Methods: Questions To Consider
While the methods described above are fairly straightforward, they are limited in scope. Often, other considerations require alterations of the simple "water-in-oil" and "oil-in-water" techniques. These considerations generally fall into one of three catagories: the desired timing and method of the release, a need for capsule content and the cell walls with special characteristics and the final destination of the microsphere.
Inducing and Controlling Microcapsule Release
The timing of the release of a microsphere depends both on the type of wall material used and, in the case of prolonged release, on the extent of cross-linking and layering between the molecules of the surface material.
For a simple pressure, temperature or chemical release, a material must be found that will break at the desired point, but no sooner. Microcapsules coating scratch ‘n’ sniff stickers must rupture only when scratched, not when pressed (for example, between the pages of a book). Chemicals used in ready-to-bake foods must rupture at high temperature, but remain whole, possibly for months, at room temperature. Capsules in instant pudding mixes and detergents must dissolve readily in water, but remain a fine, dry powder until then. Today, whole companies exsist for the express purpose of providing a microencapsultion advising service, helping other companies determine which microcapsule is best suited for their particular product, and often, going on to microencapsulate the products for their customers.
Designing a microcapsule that will release the core material slowly over a period of time is a much more complicated process than designing a capsule made to rupture, melt or dissolve. Something as simple as a change in temperature or stirring speed of a specific technique can have dramatic consequences on the cell wall, changing both the rate and efficiency of content diffusion. Usually, these manipulations of the known techniques bring about changes in one of two areas: the thickness of the cell wall or the density of its membrane (due, for example, to cross-linking).
Not surprisingly, the medical literature contains one of the most extensive discussions of ways to manipulate the timing of the core-material release. The ability to manipulate the exact timing of the release of a microcapsule’s contents is especially critical in this arena, where a slight variation of the release rate could make the drug ineffective or even lethal. Of particular importance to this release rate are factors that affect the cross-linking of the proteins (such as albumin) that are commonly used in capsule wall formation. In the process of encapsulating a drug inside a protein membrane, numerous small, individual proteins must be linked to form a continuous protein sheath. This process involves the formation of hydrogen bonds, disulfide bonds and/or ionic bonds between the individual protein molecules (Figure 3).
 |
| Figure 3. Protein Cross-linking: Disulfide Bridge Formation |
Various methods have been found for influencing the extent of cross-linking between proteins and the thickness of the resulting membranes. As early as 1972, the rates of solubilization (core material release and biodegration) were found to be related to the temperature at which the cross-linking of microspheres was induced8 (Table I). Not only did a temperature increase of just 55° C prolong the release period to 30 times the original,9 but increasing the concentration of the albumin protein has also been found to increase the density of the capsule walls, making it more difficult for core particles to get through and thus slowing the rate of release of the core material.
| Cross-linking temperature °C | 50% Solubilization time in body fluids |
|---|---|
| 135 | 24 hours |
| 160 | 84 hours |
| 170 | 4 days |
| 190 | 30 days |
Characteristics of the Capsule and its Contents
As mentioned earlier, different methods are needed to encapsulate different kinds of contents. Occasionally, reactions between the core material and the desired coating must be taken into consideration, but generally this question is limited to whether the core and coating are hydrophobic or hydrophilic. If one is hydrophobic and the other hydrophilic, some variation of the methods described earlier will usually suffice. Sometimes, however, special techniques are needed. Albumin microsphere formation provides an easily understood example of what such variations would look like, and why they might be needed.
The need for methods other than those previously described begins with an unexpected problem that occurs when the hydrophilic albumin protein matrix picks up unwanted oil from the oil bath it is injected into, turning the otherwise hydrophilic microcapsule into a slightly less hydrophilic, or even hydrophobic, microsphere. One variation on albumin microsphere formation solves this problem by using a wall polymer solution that is already greatly concentrated before injection into the oil bath and by waiting until the solution is in the oil to add any necessary cross-linking inducing chemicals.11 Another problem is encountered when the drug to be encapsulated is hydrophilic rather than hydrophobic — a concern resulting in its own set of technique variations.12
A Question of Destination: Size Manipulation of Microspheres
Size is certainly a consideration for any type of microencapsulation. If texture is important, large microcapsules can produce a grainy powder while small microcapsules produce a very fine, soft powder. Smaller particles also have a higher release rate and total release percentage per weight than do larger particles (due to a higher surface area to volume ratio). However, large particles are easier to produce.
Extensive research has been done in the last twenty years on the medical implications of microcapsule size. This is of special significance since because while microencapsulated drugs and enzymes are usually designed to treat a specific area of the body, it is often difficult to reach the target area directly. It is much more convenient to route the product through the blood. However, doing so creates the possibility of the drug spreading out through all parts of the body, with the risk of becoming diluted, or even seriously endangering the body. Size control provides an easy, effective way to target several systems, specifically the endocrine system and the lungs. Particles with a diameter of 10 m m or greater will be carried in the blood, pass through the heart and eventually become entrapped in the lungs at a rate of nearly 100%.13 Small particles (approximately 1 to 3m m in size) will pass through the lungs, depositing at a rate of nearly 90% in the liver.13 Finally, particles smaller than 1 m m will be distrubuted in the liver (80 to 90%), the spleen (5 to 8 %) and the bone marrow (1 to 2%).13 If the desired destination is, instead, a particular tissue (for example, a tumor), there are alternative methods for injecting extra large, and often hydrophobic, microcapsules directly into the nearby arteries, where they clot ("embolize") the artery and slowly release the desired drug.14
While some size-specific methods rely on filtration to retrieve capsules of a particular size from the mix of microspheres of different sizes which most methods produce, this can be costly and ineffective when the material to be incapsulated is a rare drug. Instead, it is often more effective to adjust the procedure to produce a carefully narrowed range of particle size. This can be done simply by tightening temperature controls and changes in the speeds and methods of stirring used during the coacervation process,15 but can be affected by many other factors as well (Table II).
| Factor | Change | Mean diameter |
|---|---|---|
| Oil viscosity | Decrease | Increase |
| Oil amount | Increase | Increase |
| Protein amount | Increase | Increase |
| Aqueous phase | Increase | Increase |
| Stirring phase | Increase | Decrease |
Other Considerations
In addition to the factors of release, materials and size mentioned above, it is important to take into account the yield that results from each method and the resulting cost effectiveness. How many of the microcapsules actually attain the desired size? What percentage of the core material is actually enclosed within the capsules? How much of that material is released upon activation?
All of these questions have brought the industry of microencapsulation to the level of a highly refined art — adjusted and varied to fit each particular need and application. While the concepts are the same, no two methods are exactly alike — and it’s up to the scientists to determine the best existing method for their products or to develop a method of their own.
Today, the field of microencapsulation technologies continues to grow. A simple internet search yields lists of companies ready to encapsulate almost any product. Men and women from across the world convene to discuss the field’s latest developments. New articles are published so often that an entire journal, the Journal of the Controlled Release Society (CRS), is devoted solely to the subject. The society that produces it spans the globe and includes several university level student chapters.
As we enter the new millenium, it is exciting to think what additional opportunities new microencapsulation technologies may bring. Already, complex combinations of proteins have been able to be encapsulated, forming artificial "cells" with extensive medical applications. Recent techniques have been developed for magnetizing capsule walls, allowing microencapsulated drugs to be directed to a particular location in the body by means of an artificial magnetic field. Medical applications alone include more than 90 drugs enclosed in 25 different materials,16 with applications ranging from radiation therapy for cancer to experimental treatments for AIDS. Microcapsules play important roles in every area of life, from industrial applications in adhesives and fire retardants to pesticides, food preservatives, cosmetics and photographic development. The list continues to grow, waiting only for the next generation of scientists to carry it into unchartered territories of design and application.
Endnotes
Bibliography
Discusses formation methods and applications for microcapsules containing multienzyme systems. Includes a general formation procedure and several variations, and discusses the advantages of microencapsulated of enzymes (recycling of enzymes, prevention of degradation due to environment, etc) and concludes with applications (including tumor suppression and urea removal).
An incredibly thorough and helpful article containing an overview of microencapsulation use technologies (scratch-n-sniff, carbonless copy paper, detergent, baking mixes, medication and various technological applications), an argument for the "art" of microencapsulation, and a detailed description of the coextrusion process of microencapsulation and variations on that process.
Describes methods for the formation of ethylcellulose microcapsules, an inexpensive method for coating expensive pharmaceuticals that can then be used to target specific tissue. Mainly discusses applications for tumor fighting drugs.
Discusses in detail methods for designing microspheres to release contents at the desired rates.
Another variation on the theme of albumin microsphere encapsulation of drugs, with an in-depth discussion of issues to consider when encapsulating drugs. Source of Table II.
A description of the methods for creating fragrance samplers, including paper preparation, creation of microencapsulated fragrances, and adhesion of microcapsules to prepared paper.
A detailed overview of microencapsulation, including terminology, design, uses, release mechanisms, release rates, and a list of many common microencapsulation methods and a brief description of each.
A thorough description of methods for the formation of albumin microspheres and the effects of heat and chemical methods to control in vivo release rates of the microsphere contents; includes the batch process and chemical cross-linking methods, and two side notes of interest - radioisotope addition to microcapsule walls and magnetically responsive microspheres. This is also the source of the Evans patent information, as well as Table I.
Contains lists of uses for microcapsules, as well as the articles by Ronald J. Versic, President of R T Dodge, Co.
A major microencapsulation company, specializing in the Wuster process. Contains a list of numerous applicable articles available upon request.
The home page of the Controlled Release Society. Contains information on conferences, articles and other events focused on microencapsulation, as well as a discussion page where questions can be posted and answered. This site alone is good evidence for the far reaching effects of microencapsulation in today’s society.
Home page of Coating Place, Inc., a company specializing in encapsulation.
Home page of 3M, holder of a huge number of the microencapsulation patents and a leader in the research and application fields.
Home page of Global Encap Innovations, a Malaysian based encapsulation company.